In order for cells removed during a biopsy to be examined, they must be put on a slide, stained, and then looked at under a microscope. Cells are judged based on a number of criteria, including the size and color of the nucleus (the center of the cell that contains DNA), the appearance of the other structures in the cytoplasm (the area between the nucleus and the outer wall of the cell), and the overall size of the cell itself.
For centuries, pathologists (physicians who identify diseases by studying cells and tissues under a microscope) have been trying to predict how cells will behave based on how they appear. We know that cancer occurs when a cell becomes damaged. But this damage does not occur in one step. Instead, it takes a series of "mistakes" for a cell to be transformed from a normal cell into a cancer cell, which has the ability to grow uncontrollably and to live outside the organ where it started.
Because our bodies make hundreds of millions of new cells every second, there are billions of opportunities each day for mistakes to occur in the genetic code inside a cell's DNA. Most of the time our bodies are able to find and repair or destroy these mistakes, but not always. Some mistakes slip by. And when the cells that carry a genetic mistake reproduce, they are prone to make more genetic mistakes. These mistakes in the genetic code inside the DNA can be reflected in the appearance of the cell, making it look abnormal.
What the precise problem is, though, is impossible to say. Sometimes cells that look bizarre behave in a bizarre fashion. But other times cells that look very bizarre still behave fairly normally. It all depends on which genes are damaged when the cell reproduces and the microenvironment the cells live in. Further, when looking at a cell on a slide a pathologist has no way of knowing if that cell was about to be repaired or if it was going to go undetected. The pathologist also can't determine how many mistakes have occurred within the genes and thus how close the cell might be to becoming cancerous. All the pathologist can say for sure is that the cell looks abnormal and that some damage has taken place.
The problem with the current method pathologists use to examine cells is that it is very subjective. The pathologist must determine how far from normal a cell looks, yet there is a wide variation in what is considered to be normal. This is why the experience of the pathologist reading the slides is important and why it can be helpful to get a second opinion from another pathologist. It is hoped that in the near future pathologists will be able to analyze the genetic composition of the nucleus directly to determine how damaged a cell is. This will provide better information than that obtained by judging a cell's damage by how it looks.
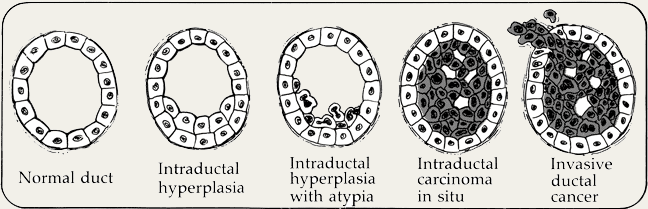
As pathologists have learned more about cells and how they change, it has become clear that what matters most is how far from normal a cell looks because the less normal the cell is in appearance, the greater the chance that genetic damage has occurred. And when genetic damage has occurred, a cell has the potential to become cancerous. This does not mean that a damaged cell will turn into a cancerous cell; it means it might. This drawing illustrates the potential progression from hyperplasia and/or atypia to invasive cancer.
Hyperplasia is the first type of abnormality in a cell's appearance. It means that there are more cells than you would expect to see in the walls of the ducts or lobules, but that all of these cells appear normal. A diagnosis of hyperplasia does not put you at any increased risk for developing breast cancer.
Atypia means that the cells look different from normal cells. You can have atypia with hyperplasia, which means that the cells look different from normal and that there are more cells than you would expect to see. You can also have atypia without having hyperplasia. A diagnosis of atypia does not mean you have cancer or even pre-cancer. It means you might have a pre-pre-cancer. In fact, most women with atypia never get breast cancer. Rather, a diagnosis of atypia means that you have a "marker" of being at increased risk for developing cancer. The increased risk for a group of women with atypia is one percent higher than it is for a group of women who do not have atypia. If a woman does not develop breast cancer in 10 years after being diagnosed with atypia, then her risk drops off significantly. In fact, it is not uncommon for a repeat biopsy in the same area of the breast to show entirely normal-appearing cells.
The terms ductal and lobular indicate where the cells originated. Ductal means that the unusual cells are in the ducts, the passages that the milk travels through to get to the nipple. Lobular means that the unusual cells are in the lobules, the parts of the breast capable of making milk. Atypia and hyperplasia are thought to be reversible, although it isn't clear what can nudge them back to normal. Atypical ductal hyperplasia (ADH) increases your risk of breast cancer occurring in the breast where the ADH was found. Atypical lobular hyperplasia (ALH) increases your risk of developing breast cancer in both breasts. Keep in mind, though, the vast majority of women diagnosed with ADH or ALH never go on to develop breast cancer.
How is atypical hyperplasia treated?
If atypical hyperplasia is diagnosed on a core biopsy, the best practice would be to have an excisional biopsy to look at the surrounding tissue and make sure this is not just the tip of the iceberg. If it was diagnosed on the basis of an excisional biopsy, you should get more details about the size and severity of what was seen.
The standard treatment for atypical hyperplasia is close follow-up. Monitoring is especially important if you have a strong family history of breast cancer. If you do, you may want to ask your doctor to recommend a program for high-risk women. These programs provide close follow-up, which means clinical breast exams every six months and yearly mammograms.
If you are diagnosed with atypical hyperplasia—ductal or lobular—your doctor may suggest that you consider taking tamoxifen. If you are postmenopausal, raloxifene (Evista) may be an option for you as well. Both drugs come in pill form and are taken daily for five years.
Tamoxifen is a type of hormone therapy routinely used to treat women with breast cancer whose tumors are hormone-sensitive. Tamoxifen has been used for breast cancer prevention since 1998, when the Breast Cancer Prevention Trial found that it reduced the incidence of breast cancer in women with atypia by 86%. What does that mean?
The Breast Cancer Prevention Trial enrolled 13,388 high-risk women. Half of the women were given tamoxifen for three to five years, while the other half were given a placebo. After following the women, researchers found 23 cases of invasive breast cancer developed in the women with atypia who had been given the placebo, while only three cases of invasive breast cancer developed in the women with atypia who were given tamoxifen. Thus, even though a risk reduction of 86% sounds large, what it actually represents is the comparison of three cases versus 23 cases.
Also, risk reduction is determined based on your original risk. What does that mean? Let's say that your risk of developing breast cancer over the next five years was 20%. An 86% reduction would be 86% of 20%, or about four percent.
Raloxifene was originally approved to treat osteoporosis. It has been recommended for postmenopausal women for breast cancer risk reduction since 2006, when the STAR (Study of Tamoxifen and Raloxifene) trial found the two drugs offered similar benefits for risk reduction. It is not safe to take tamoxifen or raloxifene if you are pregnant or trying to get pregnant. Since both tamoxifen and raloxifene have side effects, you need to discuss the risks and benefits with your physician, as the risks may outweigh the benefit you would receive.
Chemoprevention with tamoxifen or raloxifene is not a mandatory treatment. It is a choice. To decide whether it is right for you, you need to weigh the risks versus the benefits. And tamoxifen and raloxifene are not without side effects. The Breast Cancer Prevention Trial found that women taking tamoxifen had three times the chance of developing endometrial cancer (cancer of the lining of the uterus or womb) compared with women who took a placebo. Specifically, the study found that 53 of the women taking tamoxifen and 17 of the women taking the placebo developed endometrial cancer. Of these 70 cases, 67 were Stage I (which means they were caught early). So while it sounds like a large increase in the incidence of uterine cancer, the true numbers are small. Even so, it is a real risk that must be taken into consideration.
The study also found that women taking tamoxifen had twice the chance of developing a pulmonary embolism (blood clot in the lung) as women who took the placebo (28 women taking tamoxifen versus 13 on placebo). They were also more likely to develop a deep vein thrombosis (a blood clot in a major vein) than women on placebo (49 women on tamoxifen versus 34 on placebo). And they also appeared to have a slightly increased chance of stroke (71 women on tamoxifen versus 50 on placebo). Again, the numbers are small. But they are risks that must be taken into consideration.
If you have a family history of breast cancer in addition to ADH or ALH and you want to understand more about whether your family history may contribute to your breast cancer risk, you should make an appointment with a genetic counselor to discuss testing for the hereditary breast cancer gene mutations, called BRCA1 and BRCA2, which put women at higher risk for breast and ovarian cancer. The National Cancer Institute and the National Society of Genetic Counselors can help you locate a genetic counselor near you. Under the Affordable Care Act, genetic counseling and testing are covered for high-risk women.
If you decide to have genetic testing and if you are found to carry one of the BRCA gene mutations that put women at higher risk for breast and ovarian cancer, your doctor may suggest that you consider a bilateral prophylactic mastectomy (removal of both breasts). This will reduce the chance of getting breast cancer by about 95%. The surgery is only recommended if you have a strong family history of the disease. It is not recommended for women just because they have had a diagnosis of atypical hyperplasia.